Gene Detail
Contact
Missing content? – Request curation!
Request curation for specific Genes, Variants, or PubMed publications.
Have questions, comments, or suggestions? - Let us know!
Email us at : ckbsupport@jax.org
| Gene Symbol | EGFR | ||||||||||
| Synonyms | ERBB | ERBB1 | ERRP | HER1 | mENA | NISBD2 | PIG61 | ||||||||||
| Gene Description | EGFR (HER1), epidermal growth factor receptor, is a tyrosine kinase receptor, which activates RAS/RAF/MEK and PI3K/AKT/mTOR pathways, leading to increased cell proliferation and growth (PMID: 24312144). EGFR activating mutations, amplification, and overexpression are found in a variety of tumors, including non-small cell lung cancer (PMID: 26609494, PMID: 30284706) and colorectal cancer (PMID: 30243897), and the EGFRvIII variant is commonly found in glioblastoma (PMID: 30201736). | ||||||||||
|
|||||||||||
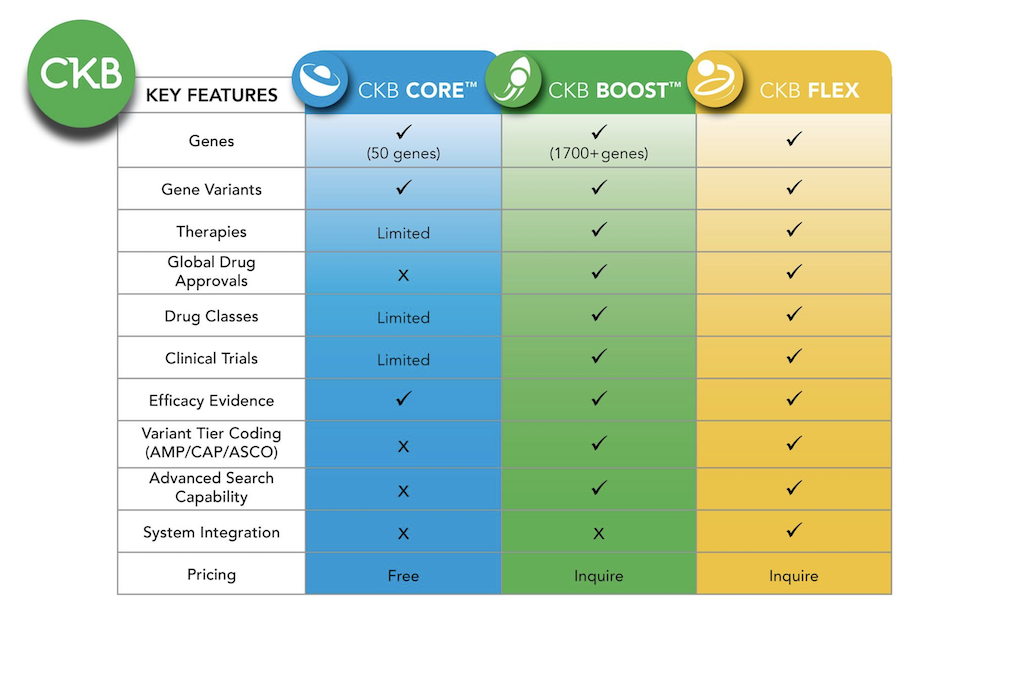
Additional content available in  CKB BOOST
CKB BOOST