Gene Detail
Contact
Missing content? – Request curation!
Request curation for specific Genes, Variants, or PubMed publications.
Have questions, comments, or suggestions? - Let us know!
Email us at : ckbsupport@jax.org
| Gene Symbol | CBLC | ||||||||||
| Synonyms | CBL-3 | CBL-SL | RNF57 | ||||||||||
| Gene Description | CBLC, Cbl proto-oncogene C, is an ubiquitin ligase that targets activated receptor kinases for degradation (PMID: 10362357). Overexpression of Cblc has been observed in non-small cell lung cancer (PMID: 29945960), mutations have been identified in myeloproliferative neoplasms (PMID: 22315494), and Cblc may modulate response to PARP inhibitors (PMID: 25883215). | ||||||||||
|
|||||||||||
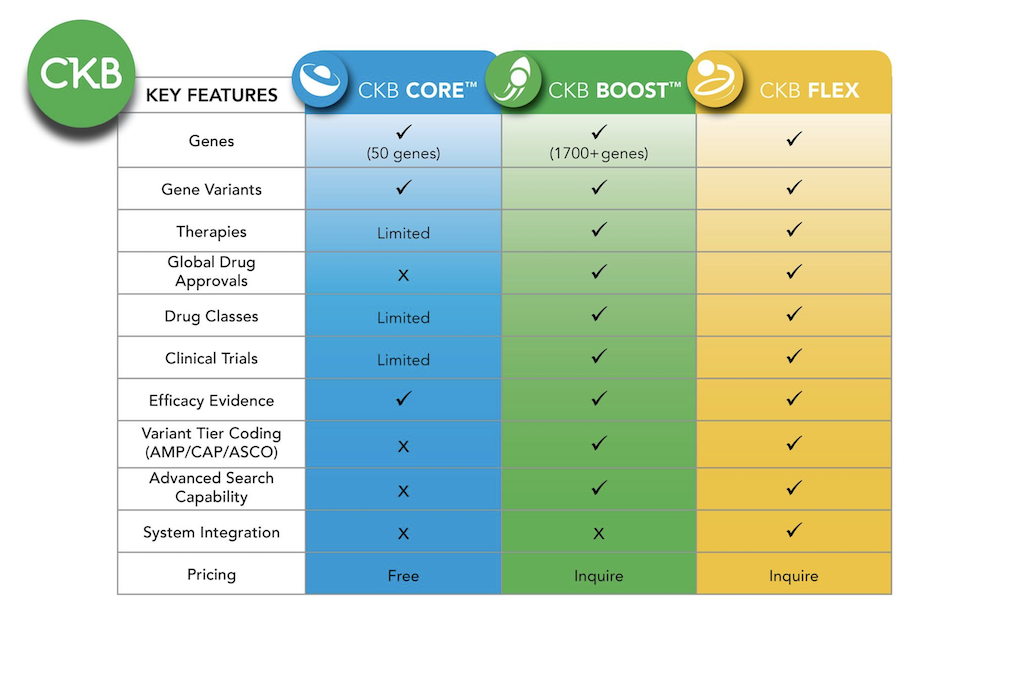
Additional content available in  CKB BOOST
CKB BOOST