Gene Detail
Contact
Missing content? – Request curation!
Request curation for specific Genes, Variants, or PubMed publications.
Have questions, comments, or suggestions? - Let us know!
Email us at : ckbsupport@jax.org
| Gene Symbol | RAD54L | ||||||||||
| Synonyms | hHR54 | HR54 | hRAD54 | RAD54A | ||||||||||
| Gene Description | RAD54L, RAD54-like, is a member of the SWI2/SNF2 family of dsDNA-dependent ATPases, which functions in homologous recombination in DNA repair (PMID: 20089461, PMID: 21704205). Overexpression of Rad54l has been demonstrated in non-small cell lung cancer (PMID: 21412013). | ||||||||||
|
|||||||||||
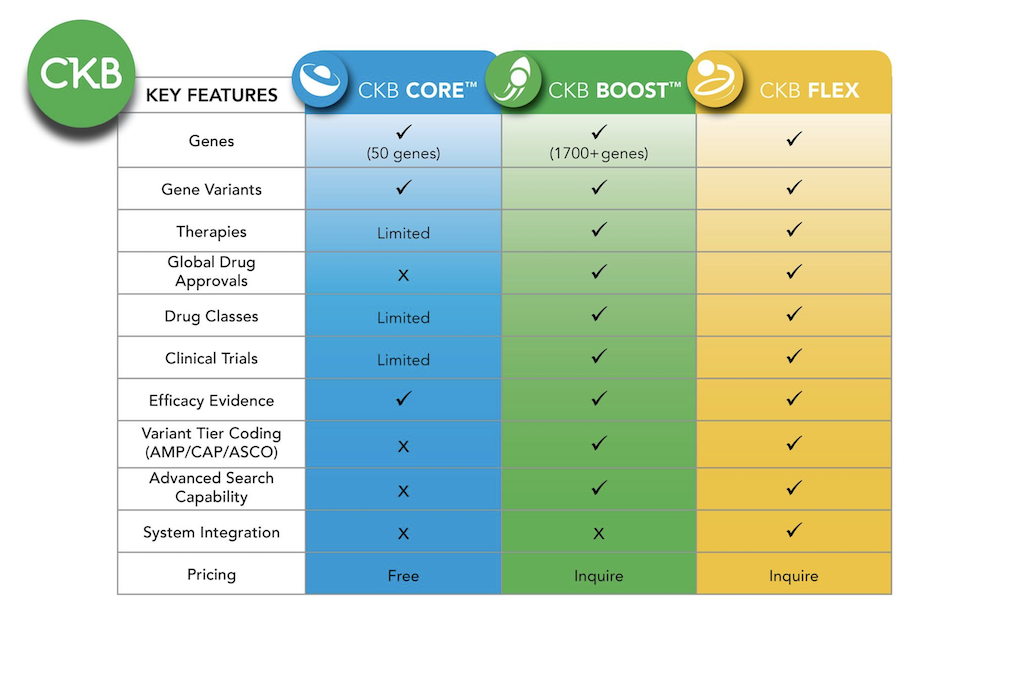
Additional content available in  CKB BOOST
CKB BOOST